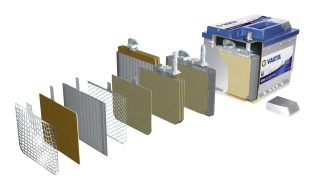
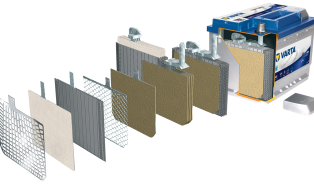
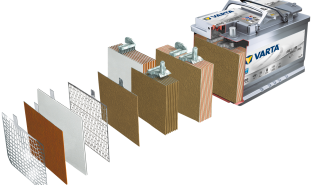
The starter battery (SLI) is a lead-acid battery that acts as a reservoir that stores electrical energy in chemical form so that it can be returned at any time.
The starter battery is responsible for two essential functions:
- provides the engine starting energy;
- allows powering electrical consumers both while the engine is running and when it is off.
Car batteries are designed to be fully charged when the car is started; after the vehicle is started, the lost charge, usually between 2% and 5% of the charge, is replaced by the alternator and the battery remains fully charged. Due to their dedicated construction (multiple and thin plates, necessary to develop high short-term currents) this type of batteries cannot be completely discharged without the risk of irreversible damage. Their number of cycles for discharges above 50% is limited to approximately 50 cycles.
A starter battery is usually constructed of “n” positive plates alternating with “n+1” negative plates, insulated from each other by microporous PVC membranes called “separators”.
The plates are connected together in parallel to form elements of much higher capacities, which are further mounted in series in polypropylene tanks and immersed in electrolyte (sulfuric acid + water).
The plates consist of grids on which the active substance is applied, which differs depending on their polarity.
GRATINGS (Fig.1) are made by casting or by expansion technology (only for lead-calcium alloys). Depending on the field of use, the thickness of the gratings varies, which makes it possible to manufacture batteries that have different technical characteristics with the same overall dimensions. Finer grating casting allows for more plates to be mounted within the same element. A larger number of plates means a larger reaction surface, which will improve the starting current. The capacity of the battery is directly proportional to the amount of active mass found on the grids. Theoretically, it has been established that for the production of 1Ah, 4.46 g of PbO2, 3.78 g of spongy Pb and 3.66 g of sulfuric acid solution are required, and the electromotive force on one element (at a concentration of 37% sulfuric acid = 1.28kg/liter) is 2.1V.
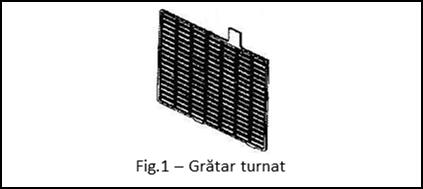
The gratings have a dual role: as a support for the active substance (PbO2 for the positive plate and spongy Pb for the negative plate) and to collect and conduct the electric current discharged by the battery.
The PLATES are based on lead alloy grids (usually Antimony or Calcium), on which the active substances are deposited differentially through the pasting / pickling process:
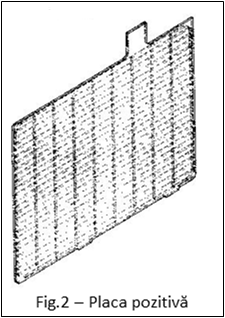
- positive plate (Fig. 2) which consists of a grid coated with PbO2 (lead dioxide) as the active substance. After the active substance, in a dry state (before charging the battery), it has a brown color, and after contact with the electrolyte it becomes reddish-brown (a sulfated or worn positive plate becomes reddish-brown or black, and the paste on it falls off when touched by an object).
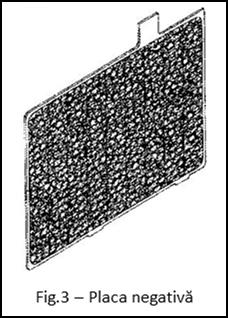
- negative plate (Fig.3) which consists of a grating on which spongy Pb is deposited. After charging the battery with electrolyte, it has the dark gray color characteristic of lead; a good plate, in contact with the electrolyte, shows a metallic luster when scratched with a hard object.
ALLOYS used for casting gratings are:
- Lead – Antimony: very good mechanical resistance, low water consumption for a lead-antimony alloy of max 3% antimony, low self-discharge, good recharge.
- Lead – Calcium: the battery does not consume water and practically does not self-discharge. Average recharge. Lower mechanical resistance.
- Hybrid: positive plates cast from Pb alloy with antimony, and negative plates expanded or cast from Pb-calcium alloy. Very low water consumption, low self-discharge, good recharge.
Due to the fact that lead is a soft metal, battery manufacturers have tried to alloy it with various chemical elements (antimony and calcium) to increase mechanical strength and corrosion resistance.
Lead-antimony batteries
Because lead is a material that does not have good mechanical resistance, the battery grids were alloyed with antimony. As a result of this alloying, which could reach up to 12% antimony, the plates became more resistant to corrosion. The major disadvantage of these batteries is that at the end of the charging process, a toxic gas is released, antimony (SbH3), which is very unstable and decomposes into hydrogen, which is released, and antimony, which is deposited on the negative plate. As a result of this, the voltage drops by approximately 0.2V, which causes the electrolysis phenomenon to occur earlier during charging and this leads to water consumption. All batteries built on Lead-Antimony technology require maintenance and the electrolyte level must be periodically topped up with distilled water, and the storage life is short due to the high self-discharge rate (approx. 10%/month). By reducing the amount of antimony in the alloy to approximately 1-3%, water consumption is reduced to 4g/Ah, which according to EN50342-1 classifies the batteries as low maintenance.
Lead-calcium batteries
Due to the disadvantages caused by lead-antimony batteries, manufacturers have replaced the lead-antimony alloy with lead-calcium. By alloying lead with calcium, the following advantages are obtained:
- Increases resistance to corrosion, vibration and shock;
- By adding Calcium to the alloy, the overcharge voltage increases from 14.4V to 14.8V, which means that gassing no longer occurs at the charging voltage. This has the advantage that water loss is almost non-existent below 1g/Ah, making the Lead-Calcium battery practically maintenance-free.
- Low internal resistance allowing high charge-discharge currents.
- Low self-discharge rate (max. 0.4% per day)
- However, permanent undercharging leads to sulfation of the battery and premature failure.
Hybrid batteries
They combine the advantage of deep-cycle reliability of Lead-Antimony batteries with the low self-discharge of Lead-Calcium batteries. A positive plate in a low antimony alloy (max 3%) creates fewer problems with deep discharge and a negative plate in lead-calcium halves self-discharge. The hybrid construction is especially ideal for semi-traction batteries.
SEPARATORS Essential to avoid short circuits between the plates. They determine performance characteristics, being made of microporous polypropylene. They wrap the positive plates of the battery in the form of envelopes.
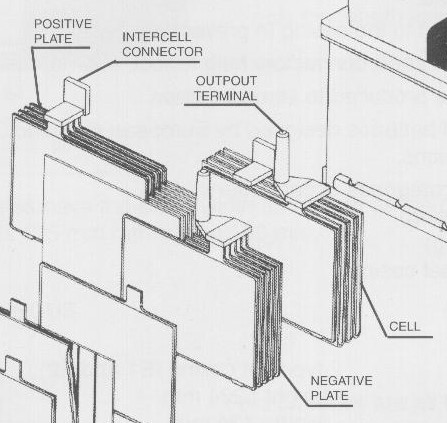
Battery CELLS are made up of negative plates and positive plates (alternating between them) fixed by connecting bridges. Gravity casting of the connecting bridges ensures high mechanical resistance of the joints between the plates and very good electrical conductivity. Heavy Duty battery cells have additional stiffening elements between the plates that form the elements, as well as between the elements and the battery, which ensures good mechanical resistance against vibrations and mechanical shocks.
The battery CASE is made of propylene (100% recyclable), thus ensuring mechanical resistance to shocks and vibrations, as well as good stability to large variations in the battery’s operating temperatures.
The battery case, through the technology by which it is manufactured, ensures 100% tightness between the battery cover and its output terminals (low risk of terminal oxidation), and the centralized ramp through which gases and electrolyte vapors are collected, together with the car’s drain hose, reduce the risks of corrosion of the bodywork.
The overall dimensions of the battery are specific to each vehicle model, but an attempt was made together with European car manufacturers to unify standards so that the battery can be mounted on as many types as possible.
CAPS
Other methods of increasing operational reliability and eliminating maintenance were obtained by constructing caps that ensure the recombination of gases (hydrogen and oxygen) and their return in the form of water to the electrolyte. Increasingly, caps are equipped with “flame arrestor” protection systems that prevent the flame from spreading inside the battery if the discharged gas mixture ignites.
The battery TERMINALS are standardized and conical; they ensure mechanical resistance and very good coupling with the vehicle’s electrical equipment.
The ELECTROLYTE is a solution of sulfuric acid and distilled water with a concentration of 37% (density 1.28 kg/liter). To reduce evaporation, elimination as gases after electrolysis, tightness against accidental breakage or for use without the risk of leakage, manufacturers have a technology for immobilizing acid in fiberglass separators (AGM batteries).
Lead-acid batteries were invented in 1859 by Gaston Planté.
In 1866, Werner von Siemens and Charles Wheatstone invented the dynamo, which transformed kinetic energy into electrical energy.
In 1871 Zénobe Gramme invented a dynamo, which made it possible for the first time to generate electricity on a commercial scale. Gramme also discovered that when two dynamos were connected in parallel, one alternator converted kinetic energy into electrical energy, and the second functioned as a motor, electrically driven by the other. With these inventions, at the end of the XIXth century, the production of electric cars was predominant. Thus, the production of batteries took on an unprecedented boost.
Classic car batteries
They are free-acid batteries, with Lead-Calcium plates with grids with a specially designed and patented geometry that increases mechanical resistance and offers better adhesion of the paste to the PowerFrame® grid.
The caps are sealed to allow gas recombination and therefore make the battery maintenance-free (except for a few models of high-capacity batteries for machinery and trucks).
Heavy duty batteries
Also, to avoid the active substance (paste) falling off the plates due to vibrations, especially for batteries used for trucks, dump trucks, tractors, and combat vehicles, the batteries have the plates fixed at the bottom as well, which is why they are distinguished from the classic ones by the name heavy duty.
EFB batteries
Developed from the need to withstand a large number of charge-discharge cycles for car batteries that equip cars with start & stop. They are batteries similar to classic car batteries in which the plates are thicker, which leads to greater adhesion of the active substance (paste) to the battery plates, providing greater reliability.
AGM batteries
They have a separator in the form of a fiberglass material (borosilicate), which is located between the electrodes and absorbs the free electrolyte, acting like a sponge. This way, the acid is more readily available to the plates, allowing for faster reactions between the acid and the plate material, allowing for higher charge/discharge rates as well as deep cycling. Another role of fiberglass separators is to promote the recombination of hydrogen and oxygen released during the charging process.
TPPL Optima accumulators
They are batteries with thin lead plates with a high level of purity (the low internal resistance allows for high current discharge), which are assembled by pressing the positive and negative plates with the associated separator, so as to obtain good fixation of the active substance on the plates as well as resistance to vibrations and deep discharges.