Unlike primary batteries, rechargeable batteries, once discharged, can be restored to their fully charged state and discharged repeatedly up to hundreds even thousands of cycles. Due to the fact that accumulators can be recharged multiple times, although more expensive than batteries, their use is more economical.
Manufacturers in the segments will focus on solutions that take into account requirements such as: price, lifespan, energy density, operational safety, etc. The increasing demand for electric vehicles produces a tremendous growth opportunity for the battery industry. Also, to meet the growing demand for energy, both industrial and domestic, we expect the demand for energy storage solutions, especially renewable energy, to increase. After a long period in which storage was done in lead-acid batteries, in the immediate future we will witness a change in type and lithium-based chemistry will probably be the future.
The diversity of batteries is very large, varying depending on chemistry, construction, or size. The choice of the optimal variant is dictated by: the discharge curve (downward or level), the self-discharge rate (loss of storage capacity – open circuit), the recharge speed (charging current compared to the battery capacity), the temperature range at which they can be used and reliability (number of cycles). No less important in choosing a battery is stability. Batteries with more active chemical elements are less stable, which implies risks in operation.
The vast majority of batteries in use are lead-acid, followed at a great distance, but on the rise, by Lithium batteries. Also, Nickel-based batteries (Nickel-Cadmium (NiCd), Nickel Hydrogen, Nickel Iron (NiFe), Nickel Metal Hydride (NiMH), Nickel Zinc) are used in applications that require high currents for short periods of time.
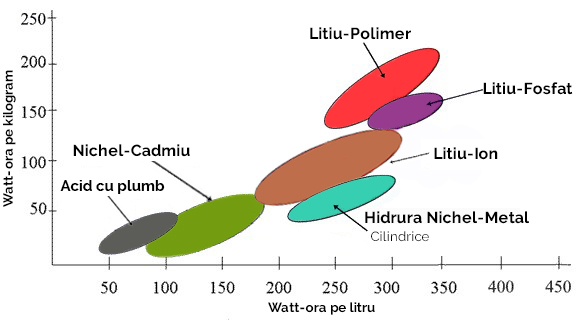
A comparison of the energy and power densities of different chemistries is shown in the following graph.
The performances of different battery chemistries are presented in the following table.
| Chemistry | Voltage | Cycles | Energy density | Power | |
| (V) | (Wh/kg) | (Wh/l) | (W/kg) | ||
| Pb/PbO2 | 2.01 | 15-1500 | 52 | 120 | 435 |
| Ni/Cd | 1.25 | 1000 | 60 | 120 | 210 |
| Zn/MnO2 | 1.25 | 55 | 60 | 130 | n/a |
| Ni/Zn | 1.6 | 400 | 70 | 130 | 260 |
| Ni/MH | 1.25 | 1000 | 80 | 240 | n/a |
| Li/VOx | 3.0 | 1000 | 93 | 115 | n/a |
| Li/V6O13 | 3.0 | 40 | 100 | 180 | n/a |
| Zn/AgO | 1.80 | 100 | 120 | 240 | 400 |
| Li/MnO2 | 2.8 | 500 | 125 | 240 | 200 |
The data in the table are data obtained under laboratory conditions.
Battery range





